Vaccine Arrival
The state of Nebraska received its first shipments of the COVID-19 vaccine during the week of December 13th, 2020. The first two shipments of vaccine to the TRPHD district were allocated to Community Action Partnership of Mid-NE, area hospitals, and TRPHD in the following total amounts:
Pfizer 460
Moderna 2000
TRPHD identified locations across the district with ultra-low temperature freezers to receive the the Pfizer vaccine. The Moderna vaccine does not need to be stored at ultra-cold temperatures, allowing it to be distributed even farther, especially to rural areas.
The first shipment to Nebraska was 13,254 doses of the Pfizer vaccine. The second shipment was 17,750 doses of the Moderna vaccine. The state determined the number of doses going to each location by surveying facilities’ needs for Phase 1. Many of the doses allocated to local public health agencies are being administered to health care workers through partnership with local facilities and Community Action Partnership of Mid-Nebraska.
Saftey and Effectiveness
Like all vaccines, COVID-19 vaccines must meet safety and effectiveness requirements set by the Food and Drug Administration (FDA) before they are made available to the general public. Vaccines undergo a rigorous scientific process that requires three phases of clinical trials before they can be submitted to the FDA for approval. In certain emergency situations, the FDA may issue an Emergency Use Authorization (EUA) to allow an investigational vaccine to be available to the general public.
COVID-19 Vaccination is Free of Cost
Cost will not be an obstacle to getting vaccinated against COVID-19. As a condition of receiving free COVID-19 vaccines from the federal government, vaccine providers will not be allowed to charge individuals for the vaccine or administration of the vaccine. Providers will not be allowed to turn away an individual because of an inability to pay or medical coverage status.
We expect that most public and private insurance companies will cover any administration fees so that there is no cost to the person getting vaccinated. If this is not the case, or if the individual does not have health insurance, providers may seek reimbursement through the Provider Relief Fund, administered by the Health Resources and Services Administration (HRSA).
Authorization and Distribution
There are several COVID-19 vaccines currently in development. The FDA issued an emergency use authorization for the Pfizer vaccine on December 11, and the Moderna Vaccine on December 18. TRPHD's vaccine distribution plan will evolve depending on various factors, including available vaccine(s) the federal government sends to Nebraska. We are prepared for many scenarios. Vaccine distribution involves new challenges like ultra-cold storage (-70 degrees) and special handling requirements.
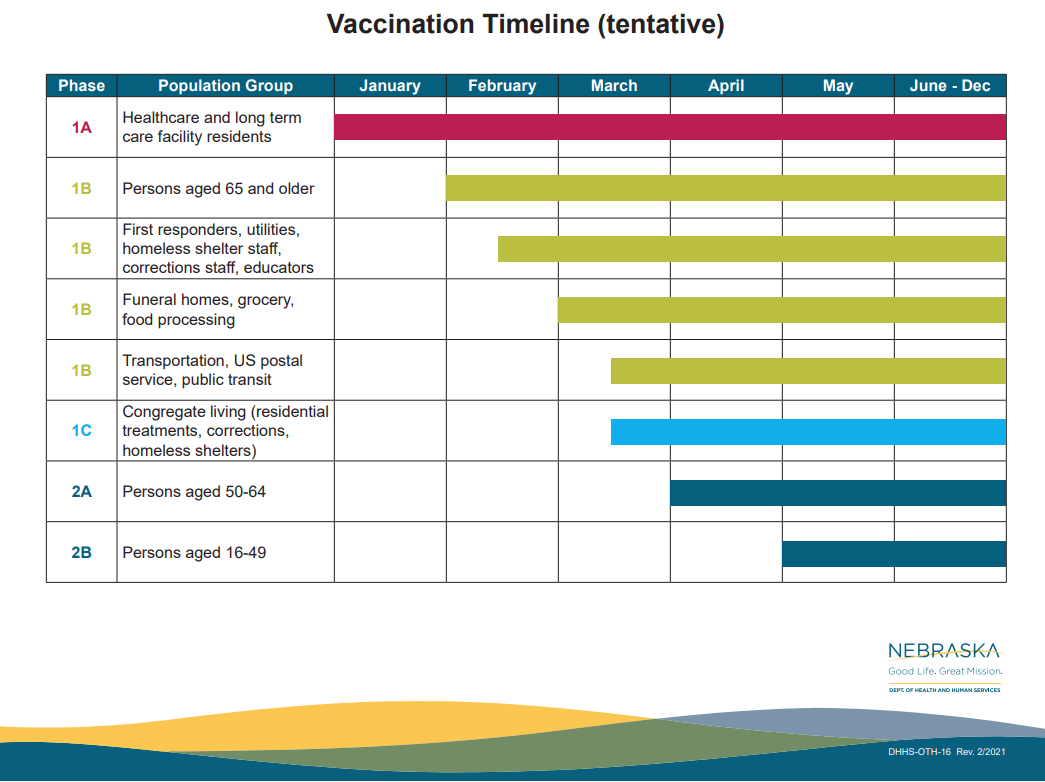
Distributing a COVID-19 vaccine to the entire population will take time. While we are planning to act swiftly, we expect it could take 6-14 months to distribute a vaccine to everyone who wants one because supplies will be limited at first. We are working closely with health care providers, pharmacies and diverse community partners to distribute the vaccine as equitably and efficiently as possible.
The State of Nebraska is distributing vaccines in phases until it is more widely available. Right now, children and pregnant women are not included in any phase, because no vaccine candidates are currently being tested in pregnant women, and only one candidate is being tested in children 12 years and older. We fully anticipate being able to vaccinate these populations once the FDA and CDC make the recommendation to do so.
Vaccine Providers
Administering the vaccine is a joint effort between public and private partners. During the initial phase, vaccine providers may include local public health agencies and large hospitals and health systems. As the vaccine becomes more widely available, the network of COVID-19 vaccine providers will expand to include doctors’ offices, pharmacies, homeless shelters, colleges and universities, senior centers, school-based health centers and other health and medical locations.
Continued Importance of COVID-19 Prevention Measures
For the foreseeable future, it will remain very important for all of us to follow public health guidance to stop the spread of COVID-19. Prevention methods still include wearing a mask in public, maintaining at least 6 feet of physical distance from others not in our household, avoiding large crowds, washing our hands often and staying home when we are sick. We ask the community to continue to follow the prevention methods in order to allow our economy to remain open while we await the vaccine.
Importance of Flu Vaccination
The flu vaccine is available now. TRPHD recommends everyone age 6 months and older get the flu vaccine as soon as possible. Getting a flu vaccine will not protect you from COVID-19, but a flu vaccination will significantly reduce your risk of getting the flu. Widespread flu vaccination will help to reduce demand on our already-stressed hospital system. Your flu vaccination status will not affect your eligibility to receive a COVDI-19 vaccine.
Additional Information and Resources
COVID-19 vaccine safety, U.S. Centers for Disease Control (CDC)
COVID-19 vaccine development and planning for Nebraska
FDA COVID-19 vaccine information
Moderna COVID19 vaccine website and factsheet, and call center for questions: 1-866-MODERNA (1-866-663-3762)